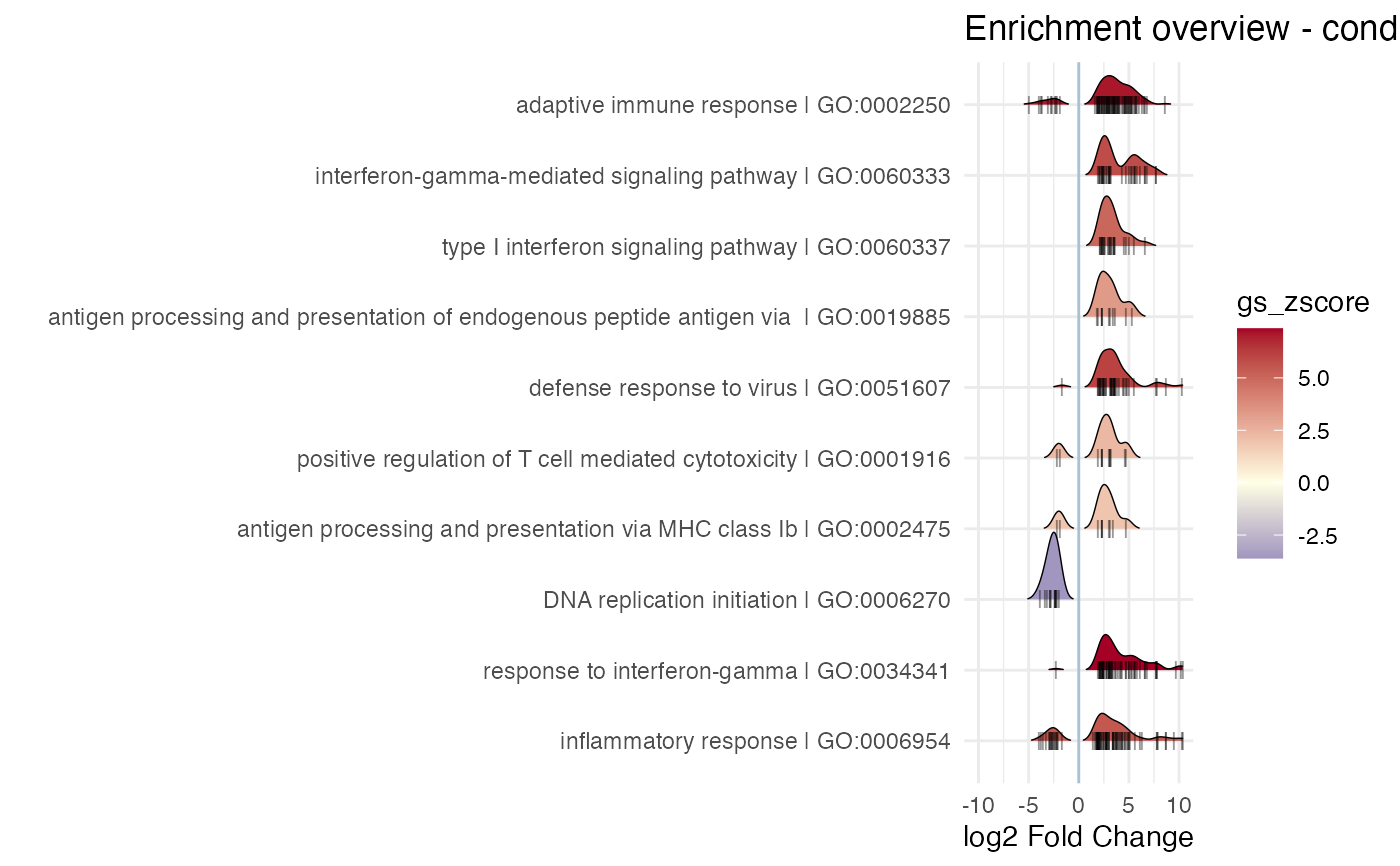
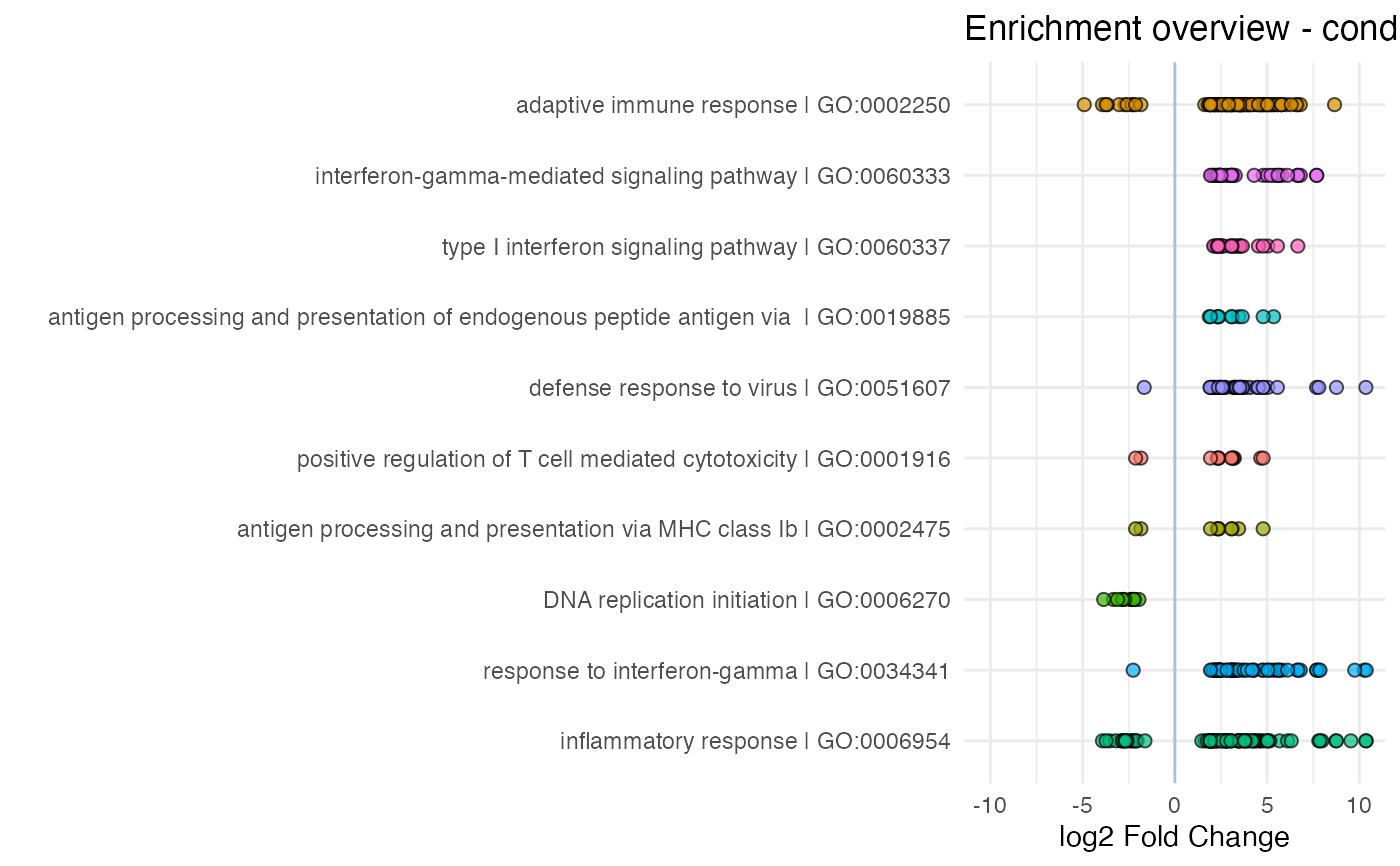
Creates a visual summary for the results of a functional enrichment analysis, by displaying also the components of each gene set and their expression change in the contrast of interest
Arguments
- res_enrich
A
data.frameobject, storing the result of the functional enrichment analysis. See more in the main function,GeneTonic(), to check the formatting requirements (a minimal set of columns should be present).- res_de
A
DESeqResultsobject.- annotation_obj
A
data.frameobject with the feature annotation. information, with at least two columns,gene_idandgene_name.- gtl
A
GeneTonic-list object, containing in its slots the arguments specified above:dds,res_de,res_enrich, andannotation_obj- the names of the list must be specified following the content they are expecting- n_gs
Integer value, corresponding to the maximal number of gene sets to be displayed.
- gs_ids
Character vector, containing a subset of
gs_idas they are available inres_enrich. Lists the gene sets to be displayed.- chars_limit
Integer, number of characters to be displayed for each geneset name.
- plot_style
Character value, one of "point" or "ridgeline". Defines the style of the plot to summarize visually the table.
- ridge_color
Character value, one of "gs_id" or "gs_score", controls the fill color of the ridge lines. If selecting "gs_score", the
z_scorecolumn must be present in the enrichment results table - seeget_aggrscores()to do that.- plot_title
Character string, used as title for the plot. If left
NULL, it defaults to a general description of the plot and of the DE contrast.
Value
A ggplot object
Examples
library("macrophage")
library("DESeq2")
library("org.Hs.eg.db")
library("AnnotationDbi")
# dds object
data("gse", package = "macrophage")
dds_macrophage <- DESeqDataSet(gse, design = ~ line + condition)
#> using counts and average transcript lengths from tximeta
rownames(dds_macrophage) <- substr(rownames(dds_macrophage), 1, 15)
dds_macrophage <- estimateSizeFactors(dds_macrophage)
#> using 'avgTxLength' from assays(dds), correcting for library size
# annotation object
anno_df <- data.frame(
gene_id = rownames(dds_macrophage),
gene_name = mapIds(org.Hs.eg.db,
keys = rownames(dds_macrophage),
column = "SYMBOL",
keytype = "ENSEMBL"
),
stringsAsFactors = FALSE,
row.names = rownames(dds_macrophage)
)
#> 'select()' returned 1:many mapping between keys and columns
# res object
data(res_de_macrophage, package = "GeneTonic")
res_de <- res_macrophage_IFNg_vs_naive
# res_enrich object
data(res_enrich_macrophage, package = "GeneTonic")
res_enrich <- shake_topGOtableResult(topgoDE_macrophage_IFNg_vs_naive)
#> Found 500 gene sets in `topGOtableResult` object.
#> Converting for usage in GeneTonic...
res_enrich <- get_aggrscores(res_enrich, res_de, anno_df)
enhance_table(res_enrich,
res_de,
anno_df,
n_gs = 10
)
 # using the ridge line as a style, also coloring by the Z score
res_enrich_withscores <- get_aggrscores(
res_enrich,
res_de,
anno_df
)
enhance_table(res_enrich_withscores,
res_de,
anno_df,
n_gs = 10,
plot_style = "ridgeline",
ridge_color = "gs_score"
)
#> Picking joint bandwidth of 0.553
# using the ridge line as a style, also coloring by the Z score
res_enrich_withscores <- get_aggrscores(
res_enrich,
res_de,
anno_df
)
enhance_table(res_enrich_withscores,
res_de,
anno_df,
n_gs = 10,
plot_style = "ridgeline",
ridge_color = "gs_score"
)
#> Picking joint bandwidth of 0.553